Abstract
Background CD3 bispecific antibodies (bsAbs) show promising clinical activity in hematologic malignancies. The tumor antigen CD30 (TNFRSF8) is highly expressed in multiple hematologic malignancies including classical Hodgkin lymphoma (cHL) and anaplastic large cell lymphoma (ALCL), while in normal tissue expression is limited to a subset of activated T- and B cells. In the last decade, treatment regimen including brentuximab vedotin have significantly improved prognosis for patients with CD30-expressing lymphomas. However, not all patients respond to treatment and many eventually develop progressive disease. Furthermore, treatment with antibody-drug conjugates and chemotherapy is associated with significant adverse events. Therefore, there is a need for better tolerated and effective treatment options for CD30-expressing lymphomas. Here, we report preclinical data for the novel DuoBody®-CD3xCD30 (GEN3017), a CD3 bsAb targeting CD30-expressing tumor cells.
Methods DuoBody-CD3xCD30 is an Fc-silenced IgG1 bispecific antibody created by controlled Fab-arm exchange of a humanized CD3ε and a human CD30 monoclonal antibody using the DuoBody® platform. The capacity of DuoBody-CD3xCD30 to induce T-cell activation, T-cell mediated cytotoxicity and production of inflammatory cytokines was analyzed in vitro and ex vivo using co-cultures of CD30-expressing tumor cells and healthy- or patient donor T cells.
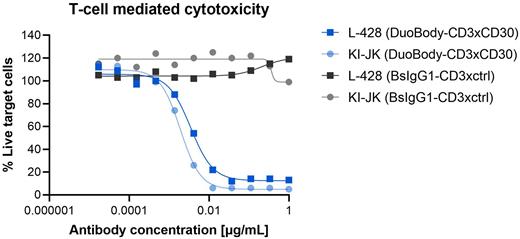
Results Expression of CD30 across different tumor cell lines of various indications was confirmed by flow cytometry. DuoBody-CD3xCD30 induced potent T-cell mediated cytotoxicity in vitro in co-cultures of T cells and CD30+ cells derived from various hematologic malignancies, including HL and ALCL, with EC50 values in the subnanomolar range (Figure 1). CD30 cell surface expression levels were positively correlated with DuoBody-CD3xCD30-induced cytotoxicity. T-cell mediated cytotoxicity was associated with T-cell activation, proliferation and cytokine production. Functional activity was dependent on crosslinking of CD3 on T cells with CD30 on tumor cells, as control antibodies targeting either CD3 or CD30 alone did not induce T-cell mediated cytotoxicity. Soluble (s)CD30 was detected in cell culture supernatant from multiple tumor cell lines and significantly correlated with CD30 membrane expression levels. The presence of sCD30 did not affect the capacity of DuoBody-CD3xCD30 to induce T-cell mediated cytotoxicity. Furthermore, CD30 expression was upregulated on healthy donor T cells upon T-cell activation. Increased CD30 expression levels was not associated with reduced viability in T-cell cultures in the presence of DuoBody-CD3xCD30. Exploratory in-depth mechanism of action studies including ex vivo functional characterization are currently ongoing and additional results will be shared.
Conclusions DuoBody-CD3xCD30 is a bispecific antibody that induces potent T-cell mediated cytotoxicity of CD30-expressing tumor cells in vitro, which was associated with induction of CD4+ and CD8+ T-cell activation, proliferation and cytokine production. These data support further clinical investigation of this novel drug candidate in CD30-expressing hematologic malignancies.
Figure 1. DuoBody-CD3xCD30 induces potent, dose-dependent T-cell mediated cytotoxicity in HL and ALCL tumor cell lines in vitro. CD30-expressing HL (L428) and ALCL (KI-JK) tumor cell lines were incubated with purified healthy donor T cells (E:T ratio = 4:1) and DuoBody-CD3xCD30 or bsIgG1-CD3xctrl for 72 hours. T-cell mediated cytotoxicity was measured by flow cytometry and expressed as the percentage viable tumor cells normalized to an untreated control sample.
Disclosures
Oostindie:Genmab: Current Employment, Current equity holder in publicly-traded company. Alemdehy:Genmab: Current Employment, Current equity holder in publicly-traded company. Janmaat:Genmab: Current Employment, Current equity holder in publicly-traded company. Meerding:Genmab: Current Employment, Current equity holder in publicly-traded company. Kemper:Genmab: Current Employment, Current equity holder in publicly-traded company. Engelberts:Genmab: Current Employment, Current equity holder in publicly-traded company. De Goeij:Genmab: Current Employment, Current equity holder in publicly-traded company. Satijn:Genmab: Current Employment, Current equity holder in publicly-traded company. Sasser:Genmab: Current Employment, Current equity holder in publicly-traded company. Breij:Genmab: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal